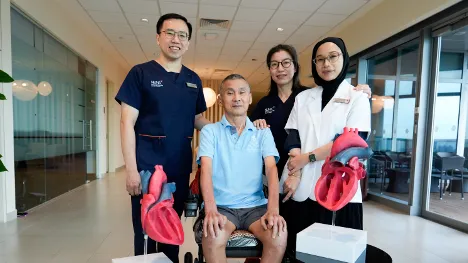
NUHCS to recruit patients for gene editing trial on rare heart condition
The disease affects around 150 patients in Singapore and can lead to heart failure.
The National University Heart Centre, Singapore (NUHCS) has become the first in Asia to recruit patients for an international clinical trial investigating a gene editing therapy for transthyretin amyloid cardiomyopathy (ATTR-CM).
The trial will evaluate the effects of the investigational gene editing therapy Nexiguran Ziclumeran (nex-z, also known as NTLA-2001) in halting the production of disease-causing proteins in affected patients.
The rare disease, which affects approximately 150 patients in Singapore, may lead to heart failure if not diagnosed and treated promptly.
Symptoms are often vague and may include numbness in the hands and feet, lethargy and dizziness.
Lin Weiqin, Clinical Director of the Heart Failure and Cardiomyopathy Programme at NUHCS, is leading the Singapore arm of the study.
“If this trial is successful, it will be the first DNA-altering treatment used in the field of adult cardiology and offers new hope to patients living with ATTR-CM,” Lin said.

















 Advertise
Advertise





