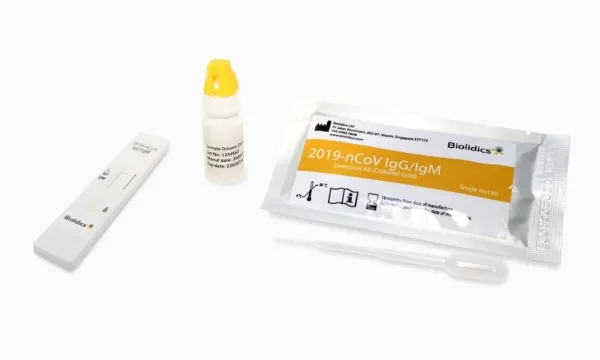
Medtech firm Biolidics allowed to sell COVID-19 test kit in EU
This followed after the test kit was allowed to be sold in Singapore and the Philippines.
Medical technology company Biolidics has been permitted to market and sell its rapid test kit for COVID-19 in the European Union (EU) after it received confirmation for a CE Marking, according to a press release.
Biolidics’ rapid test kit is one of the few that combines both IgG/IgM antibody test for COVID-19. It is generally used for detecting the presence of immune antibodies against the virus during or after infection.
The company has recently been authorised by the Philippines’ Food and Drug Administration on 31 March and provisionally by Singapore’s Health Science Authority on 27 March for its rapid test kit to be used in these countries.



















 Advertise
Advertise






