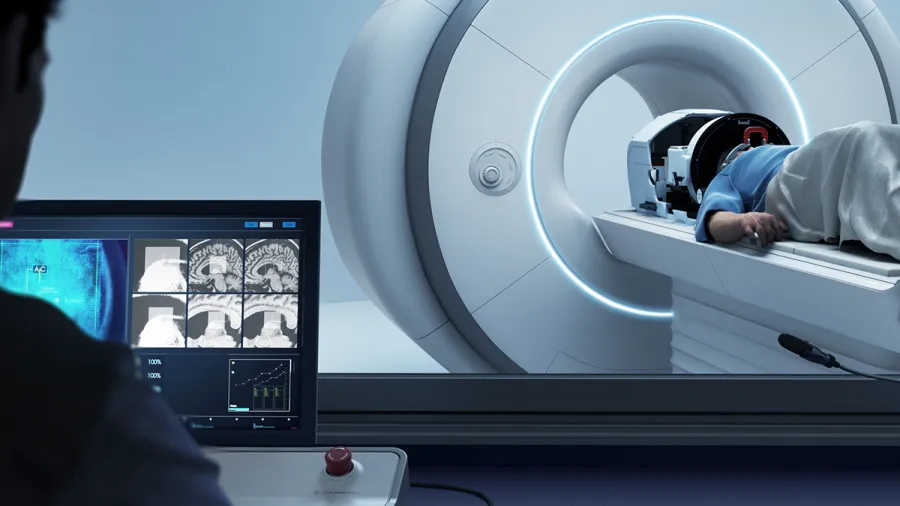
India MOH approves use of Exablate 4000 platform
This will enable the treatment of Indian essential tremors, amongst others.
Insightec announced the market approval of its incisionless neurosurgery platform, the Exablate 4000, by the Central Drugs Standard Control Organization, a part of the Ministry of Health and Family Welfare of India.
"This approval adds to the growing recognition of the value of focused ultrasound for global healthcare systems," said Insightec CEO Maurice R. Ferré.
According to Jaslok Hospital Neurosurgery Director Paresh Doshi, "This technology offers appropriate patients immediate tremor control with a less invasive procedure that requires no incisions or anaesthesia with minimal complications. Global clinical studies have demonstrated the procedure as approved is safe and helps patients regain daily function."
The Exablate 4000 platform uses MR-guided precision ultrasound to ablate small targets deep within the brain without incisions.



















 Advertise
Advertise





