China, India seen driving medical affairs outsourcing market in Asia Pacific
Outsourcing regulatory affairs services help firms save costs and focus on their core businesses.
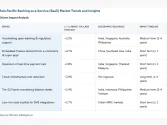
Asia Pacific’s healthcare regulatory affairs outsourcing market is projected to grow the fastest globally over the next decade led by China and India, according to a report by consultancy firm Future Market Insights (FMI).
FMI expects demand for offshore service providers assisting in regulatory processes will surge between 2023 and 2033 globally, with APAC poised to witness strong growth as more healthcare companies enter developing economies.
Its skilled workforce and cheap costs position the region as an attractive business destination according to the report.
It said China’s medical affairs outsourcing industry is on track to become a $539m market by 2033 as pharmaceutical companies expand their footprint globally, while supportive government investments and favourable healthcare policies help attract investors to set up shop in the country.
“Various pharmaceutical companies are aiming to expand their presence in China, which will require them to invest in outsourcing services for documentation, clinical trial applications, product registrations, and legal representation. This is expected to continue boosting sales in the market,” FMI said in the report.
In India, demand will likely be driven by supportive government programs like the Universal Health Insurance Scheme as well as a maturing local health tech sector.
Medical affairs outsourcing provides offshore services in securing and maintaining government approvals for drugs, medical devices and equipment.
As companies expand globally, new products are developed and costs of ramp up, FMI noted that there is an increasing need for companies to tap outsourcing services for faster documentation and approvals in their target markets as firms expand globally while there is a growing focus on novel drug development.
By segment, it said most of the demand will come from mid-size biotech and pharmaceutical firms.



















 Advertise
Advertise