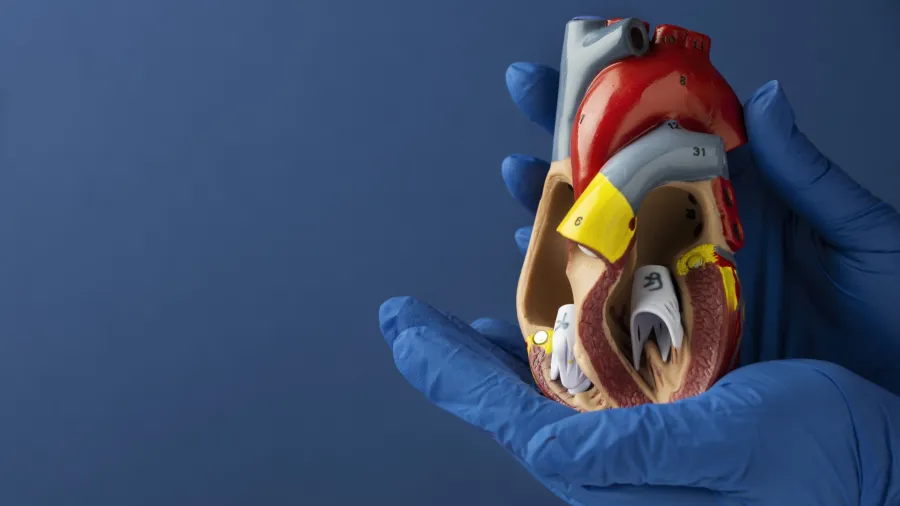
What lies ahead for Taiwan’s pacemakers market?
Adoption is also likely to accelerate across high-volume cardiac centres.
Taiwan’s pacemakers market is expected to expand at a compound annual growth rate of 3% through 2036, according to GlobalData.
The report revealed that dual‑chamber leadless pacemakers are amongst the fastest‑growing segments within cardiac rhythm management than traditional pacemakers with leads.
One notable development is the first implantations of Abbott’s AVEIR DR dual-chamber leadless pacemaker at National Taiwan University Hospital. Taiwan’s Food and Drug Administration approved the system in September 2025.
Aakansha Pankaj, Medical Devices Analyst at GlobalData, said that the technology addresses an unmet clinical need for patients requiring pacing in both chambers of the heart to manage slow or abnormal rhythms.
Adoption is also likely to accelerate across high-volume cardiac centres serving the region’s substantial annual pacemaker candidate population, the analyst added.
“Whilst premium pricing and specialised implantation training requirements may initially moderate uptake, the demonstrated reduction in procedural complications and improved patient quality of life position dual-chamber leadless pacing as the emerging standard of care for comprehensive bradycardia management,” Pankaj said.
In the Asia-Pacific, adoption of leadless pacing technology is increasing, although rates remain below those in advanced economies.
Around 25.8 million people in the region will have conditions requiring pacemakers, such as atrial fibrillation, atrioventricular block, and sick sinus syndrome, by 2035. However, current adoption rates are around 1.5–2% in Taiwan, compared with 3–5% in Germany, Japan, and the US.

















 Advertise
Advertise







